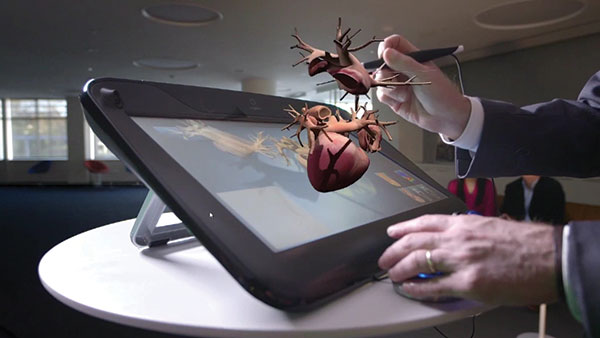
Dassault Systèmes’ Living Heart Project allows anyone to better visualize and understand how the heart functions. Image courtesy of Dassault Systèmes.
Latest in Simulate
Latest Resources

Latest Resources

Simulate Resources


Latest News
November 9, 2018
In January of next year, a team of researchers in France will launch a clinical trial that could potentially revolutionize the treatment of epilepsy. While many epileptics can be treated with medication, roughly one-third of patients suffer from pharmaco-resistant epilepsy that requires neurosurgery to control.
That surgery requires doctors to successfully locate the epileptogenic zone and remove part of the brain. However, the success rates for these surgeries have been stalled at roughly 50 to 60% for decades. Dr. Viktor Jirsa of The Virtual Brain project, and director of Research at the Centre National de la Recherche Scientifique, as well as director of the Inserm UMR1106 Institut de Neurosciences des Systèmes (INS), has found a way to improve the identification of that epileptogenic zone using personalized brain models of patients, leveraging high-performance computing (HPC) resources to conduct large-scale brain simulations.
In two pilots involving 35 patients who had already undergone surgery, the simulation approach was able to correlate the computer-predicted zone with positive surgery outcomes. The upcoming clinical trial will help guide physicians as they plan to perform surgery on several hundred patients across a number of French hospitals.

“If the pilot data holds its promise, this will mean the first improvement in decades in terms of surgery outcomes for these patients,” Jirsa says. “That is the positive predictive power of The Virtual Brain approach.”
Jirsa’s project is just one example of how simulation and modeling are gaining ground in the healthcare industry to deliver real-time patient insights using predictive analytics, and to improve the development and performance of medical devices and even drug therapies.
At the U.S. Food & Drug Administration (FDA) a number of efforts are underway to augment or potentially even replace animal testing or clinical trials with simulated trials. Eventually, that could mean medical device manufacturers could conduct much larger trials using thousands of virtual patients; in time, digital twins of specific patients could be used to simulate potential treatments both in clinical trials and in the hospital environment.
All of these efforts rely heavily on the use of HPC resources at both private and public institutions. The use of these HPC platforms has also made it possible to offer open access to many human body or human organ simulation tools so that researchers and eventually individual clinicians can leverage simulation to improve care.
Simulation and Certification
Tina Morrison, deputy director, Division of Applied Mechanics, Office of Science and Engineering Laboratories, Center for Devices and Radiological Health, at the FDA says that computational modeling has emerged as an important source of evidence when it comes to proving out new devices and treatments.
In addition to simulating the performance of a medical device, simulation and modeling could be used for assessing the safety of devices prior to clinical studies, to refine and augment clinical studies, and be used in clinical settings to help guide treatment decisions. “In theory, you could simulate a patient-specific anatomy using several different heart stints, and identify the one that would work best for the patient,” Morrison says.
Scientists can simulate actual medical devices, as well as anatomy, physiology, chemical toxicology and the manufacturing of 3D-printed devices. This could potentially increase innovation in the medical device and treatment space.
“With bench testing or animal testing, you have to minimize the number of design options to choose from because the testing is so costly,” says Steve Levine, chief strategy officer for software vendor Dassault Systèmes’ SIMULIA simulation software division. “Virtual testing opens up a whole world of innovation in the medical device field, because now you can generate an understanding of how the product will work in the context of the clinical condition it is targeted for.”
That extends into diagnostics. “You can really connect the dots between the people who are creating the solutions and those administering them,” Levine says.
The FDA has already used virtual human body models for electromagnetic, thermal and fluid dynamic simulations in evaluating more than 200 device submissions. Simulation has also been used to predict wear or corrosion in total hip replacement implants. According to Morrison, simulation has also been used to augment clinical trials for drug companies that want to more quickly test out new regimens for existing products.
By transitioning to in silico trials, companies could potentially not only save millions of dollars (a typical clinical trail costs $50,000 per patient), but also create experiments that are far more expansive than would be possible using humans or animals. A virtual clinical trial could include thousands of patients and be conducted in the course of just a few months.
Virtual Body, Organ Models
To help move healthcare simulation forward, a number of organizations have been developing virtual models of the human body and specific organs. Dassault’s Levine has played a key role in that company’s Living Heart Project, for example.
Levine’s own daughter was born with a rare heart defect, and has been living with a pacemaker since she was a toddler. It was this experience, and his frustration at the lack of data available to help improve his daughter’s treatment, that partly led to his entry into this particular field of biomedical simulation.
“We have sophisticated models that can represent an entire 10,000-part airline on a computer, but for something like the human heart we have these very simplistic models,” Levine says. “After researching the problem, we realized that a lot of it came down to the fact that the science of modeling the human body, everything was in very small pieces. Researchers in individual specialties had a lot of knowledge, but there was no vehicle to put them all together.”
Dassault Systèmes gathered this data, and used it to create a realistic model of the heart that includes the ability to simulate electrical activity, mechanics, and blood flow. First unveiled in 2015, the Living Heart is now available through the company’s 3DEXPERIENCE cloud platform.
With the model, users can convert a 2D scan of an individual heart into a personalized, fully-functional model that can be used to simulate the effects of pacemakers and surgeries.
The FDA is partnering with Dassault to help make sure the Living Heart can be safely integrated into treatment programs. The Living Heart was also used to simulate detailed drug interactions that affect organ function — researchers at Stanford used the solution for a model that can help pharmaceutical companies test drugs for the risk of inducing cardiac arrhythmias.
These complicated, multiphysics simulations still take a lot of time. It can take eight to 10 hours to simulation three cardiac cycles. A full CFD simulation can take 24 hours just to create a few heartbeats.
The EU’s Human Brain Project is another platform that has emerged to help researchers gain new understanding of how the body functions and develops. Viktor Jirsa’s Virtual Brain has also partnered with this project. Remember those snap-together Visible Man/Visible Woman models they used to sell at toy stores? There are now a number of virtual “Visible Human” projects aimed at simulating the entire body. Among them are the Visible Human Korean project, the U.S. National Library of Medicine’s (NLM) Visible Human Project, the Virtual Human Embryo at Louisiana State University, and several others.
The NLM Visible Human Project has created a complete, anatomically detailed 3D representation of both male and female bodies obtained from scans of human cadavers. Those models have been used for equipment design and surgical and diagnostic simulations.
Using these models, researchers can overcome some of the limitations of real-world clinical trials, because they can test on a virtually unlimited population of patients. However, that means that there needs to be a way to realistically represent disease states in that population. One way to do that is to use retrospective studies. If the simulations can reproduce those results, then machine learning can be leveraged to create what Levine says would be “a vast array of digital human disease states,” for use in trials.
“All of these problems are approachable,” Levine says. “We have the technology to figure it out and represent it.”
Compute-Intensive Simulations
To take full advantage of simulation in these applications, researchers have to overcome two key challenges: getting enough data about the body to create good models, and accessing a large amount of computing resources.
“You can take a car apart and test every piece,” Levine says. “You can’t do that with a human body, so a lot of the physical tests we need have to be derived in indirect ways.”
That reverse engineering problem is solvable, though, Levine says. The more complex challenge is that human bodies change over time. “That’s a different mindset than when we’re looking at something like an automobile,” he says. “The emphasis there is on what happens when the car is first created. It’s a much bigger time factor, which means we need more synergy with real-world data that we can observe. That’s where clinicians come in. They have amazing amounts of knowledge in their heads, but are not generally in the practice of translating that into paper.”
This work also requires large compute resources. “But we get better and better over time,” Levine says. “You need large numbers of cores, but this work fits within our current capabilities, and it will always get more efficient over time.”
Enabling technologies like HPC in the cloud will be critical as these efforts move from the lab to the hospital. For example, Jirsa’s epilepsy project (EPINOV) has been supported by the HPC cluster at the INS, as well as access to supercomputers in Switzerland, Germany, and Spain.
“A hospital is not going to deploy a supercomputer to do this,” Levine says. “They just want to upload images and have a heart model downloaded a few hours later onto a computer.”
Not everyone has that level of HPC power at their disposal, which is why Morrison says the FDA is working to improve access to its own HPC centers to outside stakeholders. “The theoretical aspects of these trials are straightforward, but it takes an intense level of computing power,” Morrison says.
Now that we know what is possible with simulation, Morrison suggests that the industry should do more outreach to patients so that they know what could be accomplished if more resources were committed to these efforts. “We need more resources, and getting access to data and manipulating that data to do simulations is not cheap,” Morrison says. “Maybe getting a push from the public to say they are interested in this and that they want more resources in this space could be an important launching point.”
More Dell EMC Coverage
For More Info
Dassault Systèmes
FDA
Human Brain Project
The Virtual Brain Project
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
Brian Albright is the editorial director of Digital Engineering. Contact him at de-editors@digitaleng.news.
Follow DE